

Inflammation must be controlled to improve symptoms and prevent long-term damage.2
Cycle of inflammation and infection in blepharitis3

- This inflammation affects the quality and amount of oil produced by the meibomian glands3
- Bacterial release of lipases can breakdown and interrupt the healthy, smooth oil layer3
- When there is a bacterial overgrowth on your eyelids, there may be an imbalance in the microbial population which can exacerbate symptoms3
- Bacterial release of toxins contributes to inflammation3
The American Academy of Ophthalmology recommends combination antibiotic/corticosteroid therapy for patients with blepharitis who experience acute flare-ups.4
RAPID RELIEF from Acute Blepharitis FLARE-UPS1
After 1 week of TOBRADEX® ST dosing, patients had >50% reduction in symptoms1,a
Global symptom score was determined from the following symptoms often associated with blepharitis/blepharoconjunctivitis1

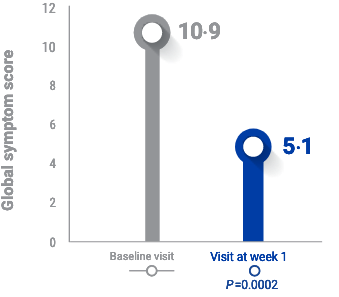
No IOP spikes were reported during the first week of treatment.1
- a Randomized, investigator-masked, active-controlled, parallel-group trial conducted at 7 private practice clinical sites in the United States with 122 adult patients who had moderate to severe blepharitis/blepharoconjunctivitis.1
- IOP=intraocular pressure.
DELIVER THE ADVANTAGES OF TOBRADEX ST WITH THE STAYING POWER OF XANGEN®5
In a preclinical study, increased viscosity improved ocular retention and bioavailability5,b
Why ST MATTERS!
The XanGen formulation of TOBRADEX ST creates a unique interaction with tears that increases the viscosity of each drop to provide longer ocular retention on the eye and increased ocular bioavailability of drug5,6,b
TOBRADEX ST suspension benefits7-9


Particles retain in lower conjunctival sac and improve drug contact time and duration of action relative to drug solution7
Consistent delivery of dexamethasone with minimal settling5
Percentage of dexamethasone per label in each drop expressed from the bottle after 24 hours5,c
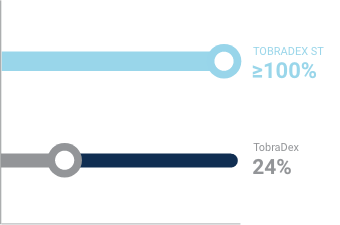

- c Based on the results of a preclinical study that examined the suspension settling characteristics of TOBRADEX ST compared to TobraDex.
- b Demonstrated in a preclinical study utilizing a rabbit model that closely mimics the human eye.5
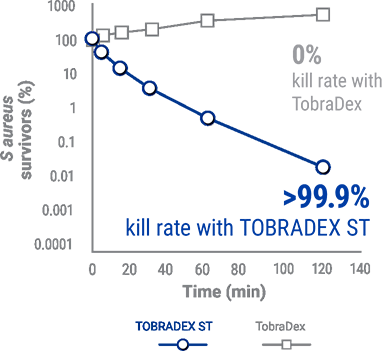
IN A PRECLINICAL STUDY, TOBRADEX®ST KILLED >99.9%
METHICILLIN-RESISTANT S. AUREUS (MRSA)5
There are no clinical studies regarding the kill rate of MRSA for TobraDex® ST.
In a preclinical study, TobraDex® showed no kill rate against MRSA.5
TobraDex® ST killed >99.9% of tobramycin- and methacillin-resistant s. aureus (MRSA).5
This information is based on preclinical data, no clinical claims are implied.
Kill rate of MRSA for TOBRADEX® ST and Tobradex®,5
Capitalize kill rate of S. Pneumoniae for TOBRADEX® ST and Tobradex®,5
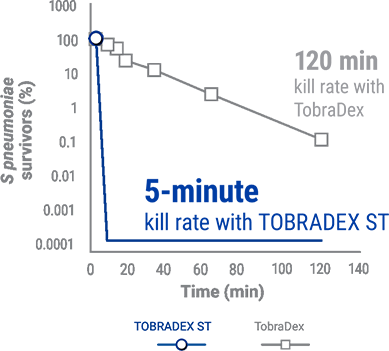
- Based on the results of a preclinical study that examined the effectiveness in killing S aureus and S. pneumoniae isolates using tobramycin concentrations measured in the tear film 10 minutes following exposure to TOBRADEX ST or TobraDex in rabbit models5
- d Multicenter, double-blind, parallel-group, single-dose study of 987 patients receiving a single dose of TOBRADEX ST or TobraDex ophthalmic suspension.5
ST MATTERS—PRESCRIBE TOBRADEX® ST
Harrow is committed to helping patients have affordable access to TOBRADEX® ST
Terms and Conditions:
-
For patients whose prescriptions are covered by commercial insurance, use of this card may reduce your copayment so that you may pay as little as $59
-
For patients whose prescriptions are not covered by either commercial insurance or Medicare, use of this card may reduce your cost for prescriptions to as little as $59
-
This card is not valid for prescriptions paid for in part or full by Medicare, Medicaid, Tricare, DOD, VA, or any state or federally funded program.
-
By enrolling in the Harrow Savings Program you certify to the following: (1) Your Medicare plan does not cover your Harrow prescription medication; (2) you will not seek any prescription coverage or reimbursement from your Medicare plan for the the cost of the Harrow prescriptions received through this offer or report any amounts paid in connection with this offer toward your True Out-of-Pocket (TrOOP) costs under your plan; (3) that you will purchase all Harrow prescriptions covered under this offer during the calendar year by using the Harrow Savings Program and will not use your Medicare benefits even if your benefits change
-
This program is subject to overall maximum support amounts.
-
This offer shall be applied only toward the cost of an eligible prescription product and not toward ancillary services or treatment costs.
-
This offer is only good in the United States of America (including the District of Columbia, Puerto Rico, Guam, and the U.S. Virgin Islands).
-
You must present this coupon along with your prescription to participate in this program.
-
This offer is not health insurance.
-
The selling, trading, or counterfeiting of this coupon is prohibited by law. Void if reproduced.
-
This offer is not transferable.
-
When you use this offer, you are certifying that you understand and agree to comply with the program rules, regulations, eligibility requirements, and Terms and Conditions.
-
Harrow Health reserves the right to rescind, revoke, or amend this offer at any time.
For questions call: 1-316-219-4495
DELIVER THE TOBRADEX® ST DIFFERENCE TO RESOLVE ACUTE FLARES
Dosing and administration6
- One drop of TOBRADEX ST should be administered to the affected eye every 4 to 6 hours
- During the initial 24 to 48 hours, dosage may be increased to one drop every 2 hours
- Frequency should be decreased gradually as clinical signs improve
- Therapy should not be discontinued prematurely
TOBRADEX ST has no therapeutic or generic equivalent
Indication and Usage
TOBRADEX® ST (tobramycin and dexamethasone ophthalmic suspension) 0.3%/0.05% is a topical antibiotic and corticosteroid combination for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Important Safety Information
CONTRAINDICATIONS:
Most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and also in mycobacterial infection of the eye and fungal disease of ocular structures. Hypersensitivity to any components of the medication.
WARNINGS & PRECAUTIONS:
- Intraocular Pressure (IOP) Increase—Prolonged use may result in glaucoma with damage to the optic nerve, and defects in visual acuity and fields of vision. IOP should be monitored.
- Aminoglycoside Sensitivity—Sensitivity to topically applied aminoglycosides may occur.
- Cataracts—Posterior subcapsular cataract formation may occur.
- Delayed Healing—May delay healing and increase the incidence of bleb formation. Perforations of the cornea or sclera have occurred. Slit lamp biomicroscopy and fluorescein staining should be conducted.
- Bacterial Infections—May suppress host response and increase secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, then the patient should be re-evaluated.
- Viral Infections—Use with a history of herpes simplex requires great caution. The course and severity of many viral infections of the eye (including herpes simplex) may be exacerbated.
- Fungal Infections—Fungal infections of the cornea may occur and should be considered in any persistent corneal ulceration.
- Vision Blurred—Vision may be temporarily blurred following dosing with TOBRADEX® ST. Care should be exercised in operating machinery or driving a motor vehicle.
- Risk of Contamination—Do not touch the dropper tip of the bottle to any surface, as this may contaminate the contents.
- Contact Lens Use—TOBRADEX® ST contains benzalkonium chloride, an antimicrobial preservative, which may be absorbed by soft contact lenses. Contact lenses should not be worn during the use of TOBRADEX® ST.
ADVERSE REACTIONS:
The most frequent adverse reactions (<4%) to topical ocular tobramycin are hypersensitivity and localized ocular toxicity, including eye pain, eyelid pruritus, eyelid edema, and conjunctival hyperemia.
The reactions due to the steroid component are increased IOP with possible damage to optic nerve and development of glaucoma, subcapsular cataract, and impaired healing.
The development of secondary infection has occurred. Fungal infections of the cornea may occur. Secondary bacterial ocular infection following suppression of host responses also occurs.
Non-ocular adverse events (0.5% to 1%) included headache and increased blood pressure.
The following additional adverse reactions have been reported with the individual components below:
- Aminoglycosides: Neurotoxicity, ototoxicity, and nephrotoxicity have occurred in patients receiving systemic aminoglycoside therapy. Aminoglycosides may aggravate muscle weakness in patients with known or suspected neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, because of their potential effect on neuromuscular function.
- Dexamethasone: Cushing’s syndrome and adrenal suppression may occur after the use of dexamethasone in excess of the listed dosing instructions in predisposed patients, including children and patients treated with CYP3A4 inhibitors.
POSTMARKETING EXPERIENCE:
The following adverse reactions have been identified during postapproval use of TOBRADEX® ST. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Additional adverse reactions identified from postapproval use include anaphylactic reaction and erythema multiforme.
Please see the full Prescribing Information.
References: 1. Torkildsen GL, Cockrum P, Meier E, et al. Evaluation of clinical efficacy and safety of tobramycin/dexamethasone ophthalmic suspension 0.3%/0.05% compared to azithromycin ophthalmic solution 1% in the treatment of moderate to severe acute blepharitis/blepharoconjunctivitis. Curr Med Res Opin. 2011;27(1):171-178. 2. Mand PM, Mannis MJ. What is the best treatment approach for severe blepharitis? Vis Pan-Am. 2014;13(3):67-69. 3. Baudouin C, Messmer EM, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100:300-306. 4. Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern. Ophthalmology. 2019;126(1):P56-P93. 5. Scoper SV, Kabat AG, Owen GR, et al. Ocular distribution, bactericidal activity and settling characteristics of TOBRADEX® ST ophthalmic suspension compared with TobraDex® ophthalmic suspension. Adv Ther. 2008;25(2):77-88. 6. TobraDex ST. Prescribing information. Eyevance Pharmaceuticals LLC; 2022. 7. Patel A, Cholkar K, Agrahari V, et al. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47-64. 8. Rozi MF, Sabere ASM. A review on conventional and novel topical ocular drug delivery system. J Pharmacy. 2021;1(1):19-26. 9. Duxfield L, Sultana R, Wang R, et al. Ocular delivery systems for topical application of anti-infective agents. Drug Dev Ind Pharm. 2016;42(1):1-11. 10. U.S. Department of Health and Human Services, Food and Drug Administration. Approved drug products with therapeutic equivalence evaluations (Orange Book). 42nd ed. Washington, DC: U.S. Department of Health and Human Services, Food and Drug Administration; 2022.

